5. Explain
6. Convert the following.
i. Propene to propan-1-ol
ii. Benzyl alcohol to benzyl cyanide
iii. Ethanol to propane nitrile
iv. But-1-ene to n-butyl iodide
v. 2-Chloropropane to propan-1-ol
vi. tert-Butyl bromide to isobutyl bromide
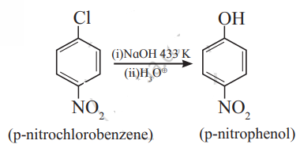
vii. p-Nitrochlorobenzene to p-nitrophenol
viii. Propene to 1-nitropropane
Answer:-
2

3) \[\ce{\underset{\text{But-1-ene}}{CH3 - CH2 - CH = CH2 + HBr}->[Peroxide] \underset{\text{1-Bromobutane}}{CH3 - CH2 - CH2 - CH2Br}}\]
4) \[\ce{\underset{\text{1-Bromobutane}}{CH3 - CH2 - CH2 - CH2Br + NaI} ->[Acetone]\underset{\text{n-Butyl iodide (1-Iodobutane)}}{CH3 - CH2 - CH2 - CH2I + NaBr↓}}\]
5)\[\begin{array}{cc}
\ce{H3C - CH - CH3 ->[Alc.KOH]\underset{Propene}{H3C - CH = CH2}->[HBr][Peroxide]\underset{1-Bromopropane}{H3C - CH2 - CH2Br}->[NaOH(aq.)]\underset{Propan-1-ol}{H3C - CH2 - CH2OH}}\\
|\phantom{...........................................................................................}\\
\ce{\underset{2-Chloropropane}{Cl}\phantom{..........................................................................................}}
\end{array}\]
6)\[\begin{array}{cc}
\ce{\phantom{.........}Br\phantom{.........................................................}H\phantom{............}}\\
\phantom{........}|\phantom{...........................................................}|\phantom{...........}\\
\ce{H3C - C - CH3 +\underset{(Alc.)}{KOH} -> H3C - C = CH2 + HBr ->[Peroxide] H3C - C - CH2Br}\\
\phantom{...}|\phantom{............................}|\phantom{...............................}|\phantom{.....}\\
\ce{\underset{\underset{(2-Bromo-2-methyl propane)}{Tert-Butyl bromide}}{CH3}\phantom{...............}\underset{\underset{(2–Methylprop-1-ene)}{Isobutene}}{CH3}\phantom{....................}\underset{Isobutyl bromide}{CH3}\phantom{...}}\\
\end{array}\]
7